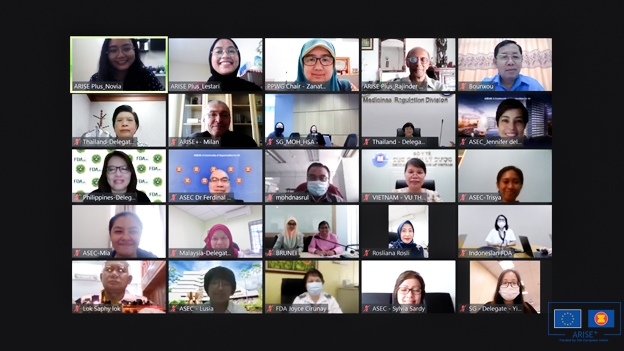
Officials from ASEAN Member States have launched a new initiative that involves active cross pillar collaboration in ensuring the quality, safety and efficacy of medicines that are placed in the ASEAN market. This collaboration has been undertaken by the ASEAN Pharmaceutical Product Working Group (PPWG) under the ASEAN Economic Ministers and Health Cluster 3 Strengthening health system and access to Care (AHC 3), a Group under the ASEAN Health Ministers.
The Workshop on ASEAN Pharmaceutical Regulatory Policy and the ASEAN Pharmaceutical Regulatory Framework (APRP and APRF) was held on the 21 – 22 April 2021 with the support of ARISE Plus to discuss the formulation of this policy and regulatory framework for ASEAN. The workshop is a first significant milestone in the ambitious longer-term target to establish and adopt a common policy that when adopted can be implemented through an ASEAN framework that will provide the structure legal instruments and implementation arrangements.
For more details, please visit: News on Workshop on the ASEAN Pharmaceutical Regulatory Policy and the ASEAN Pharmaceutical Regulatory Framework (APRP and APRF)