Jakarta, September 2020
Twenty Five (25) participants representing all Member States national drug regulatory agencies from the ASEAN Pharmaceutical Product Working Group (PPWG) participated in an online workshop to deliberate on the proposed establishment of an ASEAN Framework for Pharmaceutical Regulation. The discussions were moderated by experts from ARISE Plus with the support of 2 officials from the ASEAN secretariat.
This event was a follow-up to a discussion paper that had been developed by ARISE Plus experts in consultation with ASEAN counterparts.
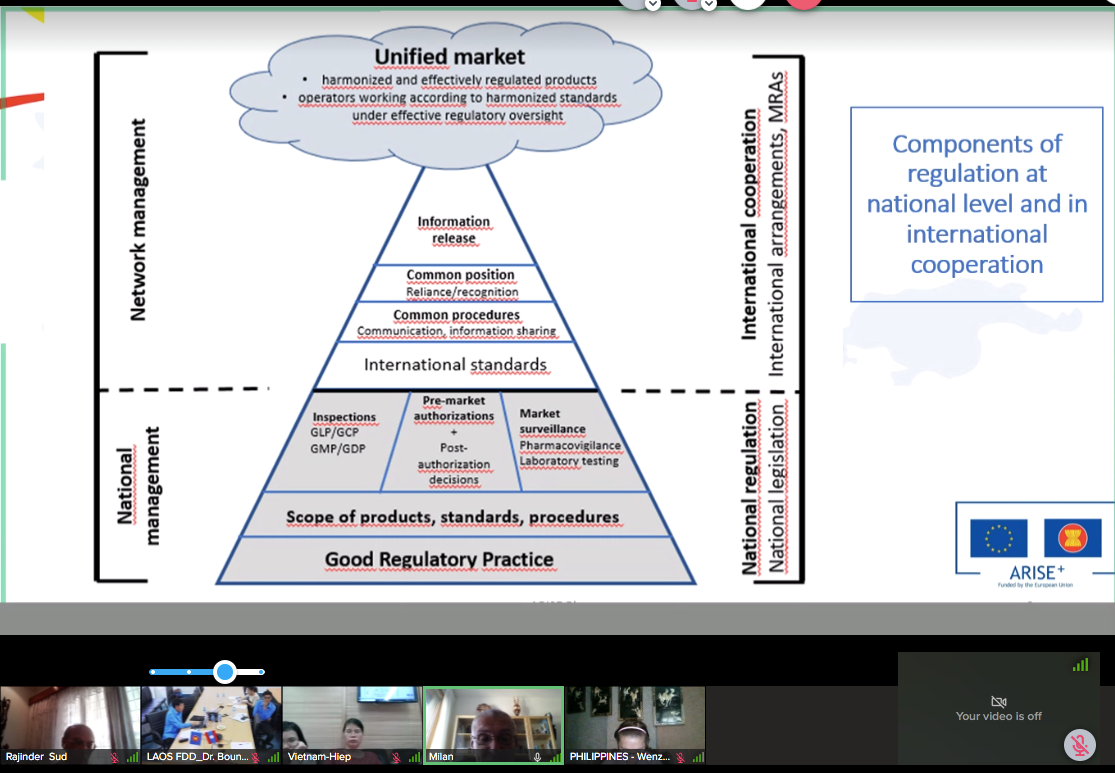
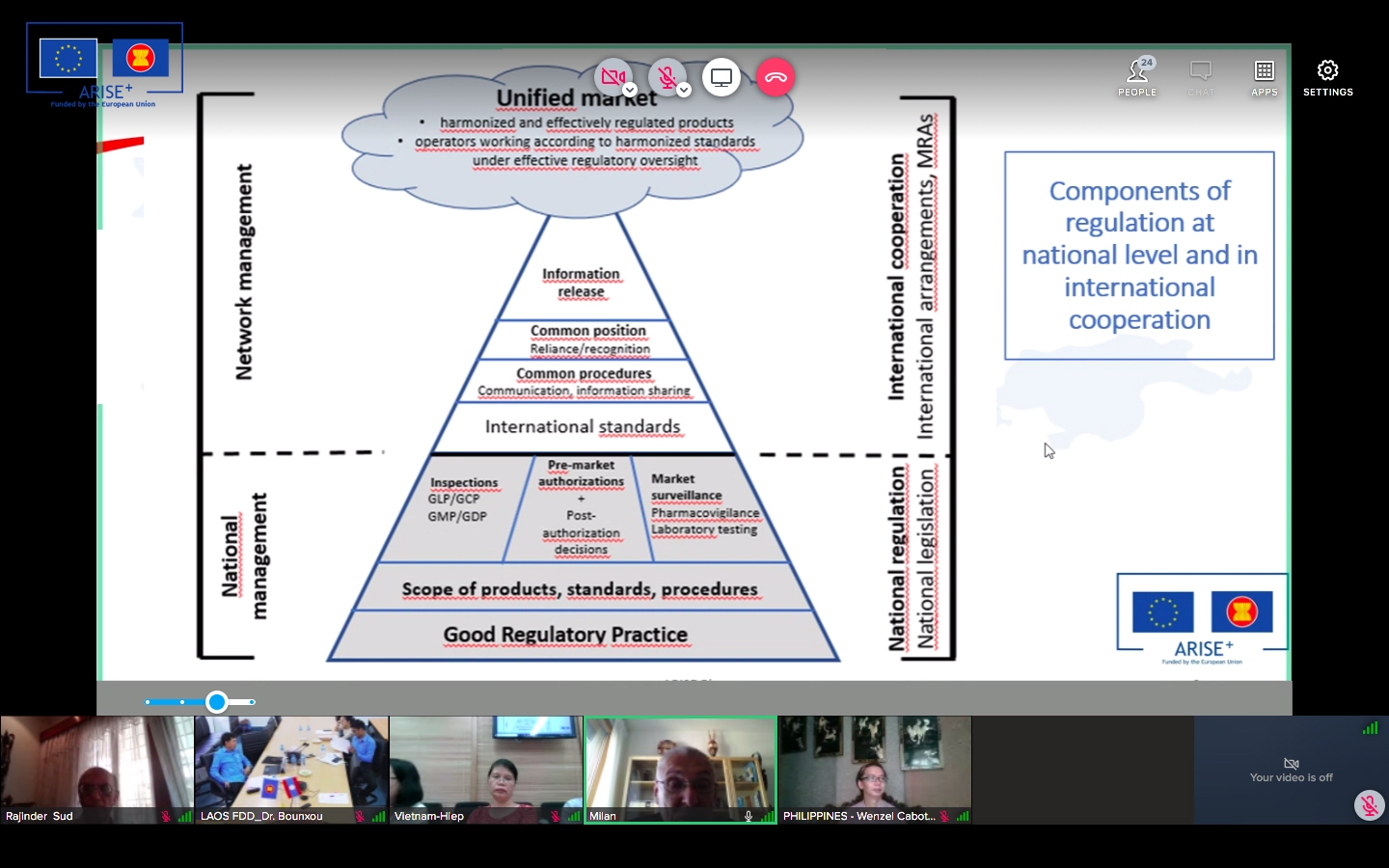
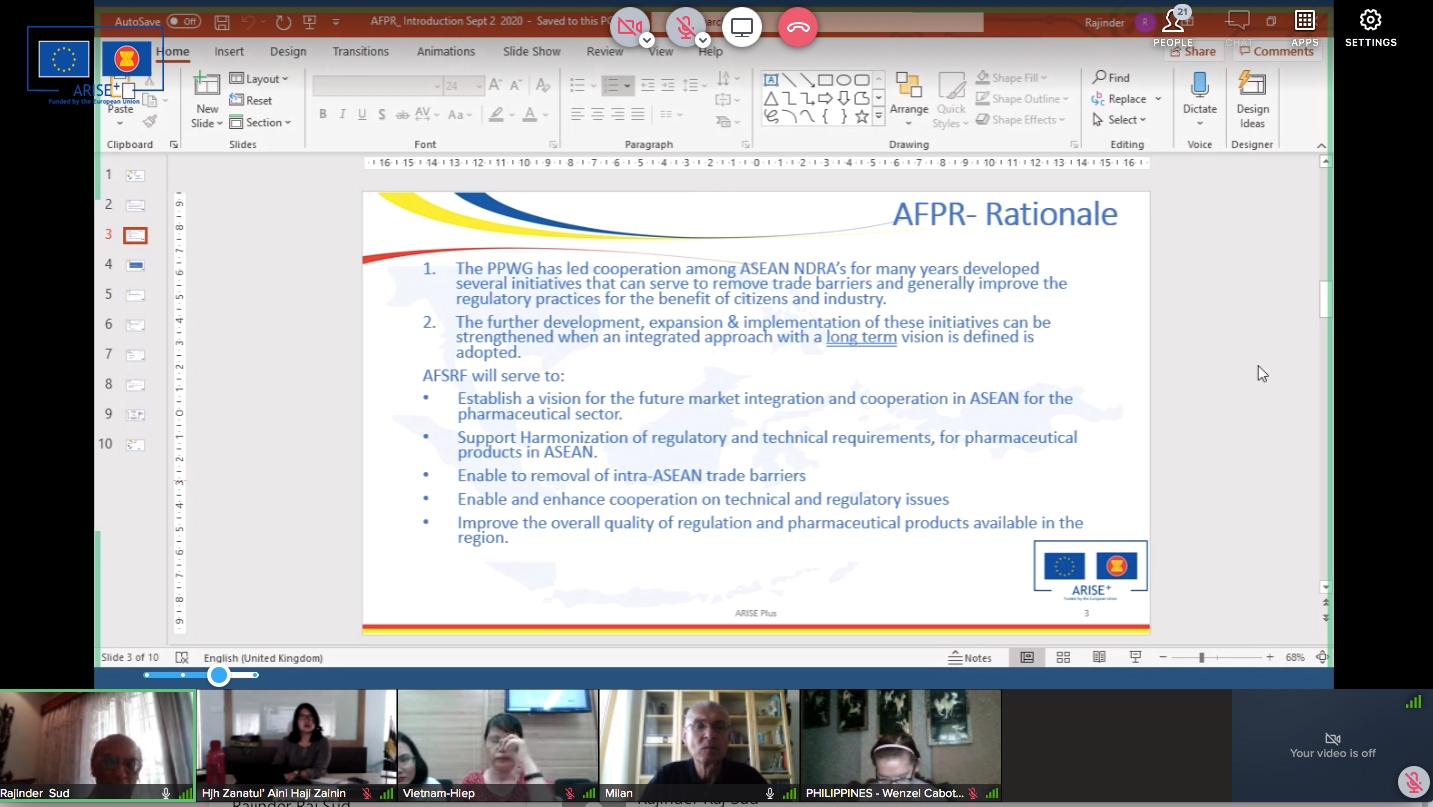
The discussion paper on the development of an ASEAN Framework for Pharmaceutical Regulation (AFPR) was circulated to PPWG Members in March 2020 with the objective of seeking the views of PPWG Members in the formulation of a draft for the establishment of an ASEAN Framework for Pharmaceutical Regulation (AFPR). The paper proposed the scope, contents and key elements of a framework that could provide a coherent and integrated approach for regulation in the sector, links existing initiatives, close gaps and facilitate effective regulation of across the ASEAN and lead to reduction of trade barriers in the pharmaceutical sector.
It is intended that the Framework will provide for a coherent and integrated approach to the ASEAN Pharma market and links the current initiatives of the PPWG ensuring that effective regulation of pharmaceuticals. The AFPR would build upon the existing commitments in order to provide a structure and the instruments to realise the free flow of safe, efficient and quality pharmaceuticals in the region. The Framework will incorporate existing ACCSQ guidelines and recognition arrangements.
Since the establishment of the PPWG by the ASEAN Consultative Committee for Standards and Quality (ACCSQ) in 1999, the PPWG has developed harmonized requirements in several important areas of pharmaceutical regulation (Good Manufacturing Practice Mutual Recognition Arrangement, ASEAN Mutual Recognition Arrangement (MRA) for Bioequivalence Study Reports of Generic Medicinal Products, ASEAN Common Technical Dossier (ACTD), ASEAN Common Technical Requirements (ACTRs),) and is implementing initiatives towards this objective (Joint Assessment project). In its current Strategic Plan, PPWG identified that it aims to it expand the scope of current recognition arrangements, and review the post-authorization initiatives.
The discussions on the 2nd September facilitated a convergence of views on the approaches towards developing the framework and included active discussions on the scope, objectives, governing principles, implementing methods and management aspects of the proposed framework. These views are now being consolidated for development of a draft of the ASEAN Framework for Pharmaceutical Regulation.